Introduction
Human skin, the body’s largest organ, is structured in layers and frequently exposed to ultraviolet radiation (UVR) from the sun, a major cause of DNA damage. This damage can occur directly through the interaction of UV photons with DNA, or indirectly, primarily via increased production of reactive oxygen species (ROS) that cause oxidative changes to DNA. Oxidative stress and DNA damage lead to significant changes at the cellular and molecular levels, affecting cell cycle and signal transduction factors involved in DNA repair, aging, and the bystander effect.
The bystander effect refers to biological changes in non-irradiated cells neighboring irradiated cells. These changes involve a complex network of interactions. Natural bioactive compounds may modulate this signaling. This article will discuss the protective applications of natural bioactive compounds, particularly in the context of skin damage.
UVR-Induced DNA Damage
Solar UVR is a major environmental factor causing DNA damage and skin photoaging. UVR includes UVB (280-315 nm) and UVA (315-400 nm), with UVA making up about 95% of all UVR. Both types penetrate the epidermis, but UVA reaches the dermis due to its deeper penetration.
UVR, especially UVB, is absorbed directly by DNA, causing damage. UV photons can induce transitions and the formation of cyclobutane pyrimidine dimers (CPD) and pyrimidine photoproducts. Indirect DNA damage also occurs when UV photons are absorbed by non-DNA chromophores, leading to ROS formation. UVA and UVB both stimulate ROS production, but UVA mainly induces the oxidative processes that have mutagenic and cytotoxic effects on DNA.
Human skin contains various non-DNA chromophores, including riboflavins, porphyrins, haem, bilirubin, melanin precursors, pterins, flavins, tryptophan, and urocanic acid. These molecules can absorb UV photons, leading to structural changes and photosensitizing reactions. These reactions can produce compounds such as superoxide, hydrogen peroxide, hydroxyl radical, and singlet oxygen. Singlet oxygen frequently oxidizes guanine in DNA to 8-oxo-7,8-dihydroguanine (8-oxoG). ROS, particularly hydroxyl radicals, also induce the formation of 8-hydroxydeoxyguanosine (8-OHdG), a major product of oxidative stress. ROS generation also leads to the oxidation of other macromolecules, further destabilizing DNA.
DNA damage responses (DDR) in skin cells counteract the harmful effects of UVR. Unrepaired DNA damage can block DNA and RNA polymerases, limiting replication and transcription. DNA damage typically limits cell proliferation through temporary cell cycle arrest, allowing for repair mechanisms to prevent the spread of mutations.
DNA Repair Mechanisms
The main mechanisms of DNA repair are base excision repair (BER) and nucleotide excision repair (NER). BER repairs small base lesions and single-strand breaks (SSB). Key enzymes involved in BER include AP-endonuclease (APE1), DNA polymerases, flap endonuclease (FENI), and DNA ligases. NER is important for repairing UVR-induced DNA damage, such as photoproducts and thymine dimers. NER is initiated by damage recognition, removal of the single-stranded fragment, synthesis of a complementary sequence, and ligation to form double-stranded DNA.
There are two forms of NER: global genomic NER (GG-NER) and transcription-coupled NER (TC-NER). GG-NER repairs damage in both transcriptionally active and silent regions of DNA, using proteins that recognize structural distortions. TC-NER repairs damage only in transcriptionally active regions, using RNA polymerase stalls for DNA damage recognition. The efficiency of both BER and NER declines with age.
DNA Damage and Age-Related Cell Signaling
Photoaging also affects mitochondrial DNA (mtDNA). UVR often causes deletion of DNA base pairs, contributing to increased ROS production by mitochondria and increased ROS-induced mtDNA damage.
In cells, ROS is produced in various organelles as a byproduct of aerobic metabolism. The electron transport chain (ETC) in the inner mitochondrial membrane uses several complexes to produce water. Electrons can also react prematurely with oxygen, leading to the formation of superoxide. Mitochondrial nitric oxide synthase (NOS) produces nitric oxide (NO), which can combine with superoxide to form peroxynitrite. Peroxisomal ROS production is driven by various enzymes and oxidoreductases. ROS can also be generated in the cytosol as a byproduct of cyclooxygenase (COX) and lipoxygenase (LOX) activity.
Hydrogen peroxide is a secondary messenger that can activate several redox-sensitive signaling molecules involved in regulating cell proliferation and migration. These include mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinases (ERK1/2), c-Jun NH2-terminal kinases (JNK 1/2/3), phosphoinositide 3-kinase/serine-threonine kinase (PI3K/Akt), protein kinase B (AKT), and transcription factors like activator protein 1 (AP-1), nuclear factor-κB (NF-κB) and early growth response 1 (Egr1). ROS-induced alterations to these signaling pathways can activate proto-oncogene pathways, relevant to skin aging.
UVR-induced ROS can trigger inflammation, oxidative stress, and DNA damage in neighboring cells via the bystander effect. Senescence, a cellular event in response to UVR damage, is associated with irreversible cell proliferation arrest and the development of a senescence-associated secretory phenotype. Increased levels of 8-OHdG, indicative of oxidative stress and DNA damage, are observed in senescent cells.
Cell proliferation is regulated by p53/Rb axis signaling. The tumor suppressor p53, a transcription factor activated by DNA damage or oxidative stress, mediates the oxidative stress response. Retinoblastoma protein (pRb) is a tumor suppressor controlling transitions from the G1 to S-phase of the cell cycle. Accumulation of p16 and p53 is associated with premature cell senescence.
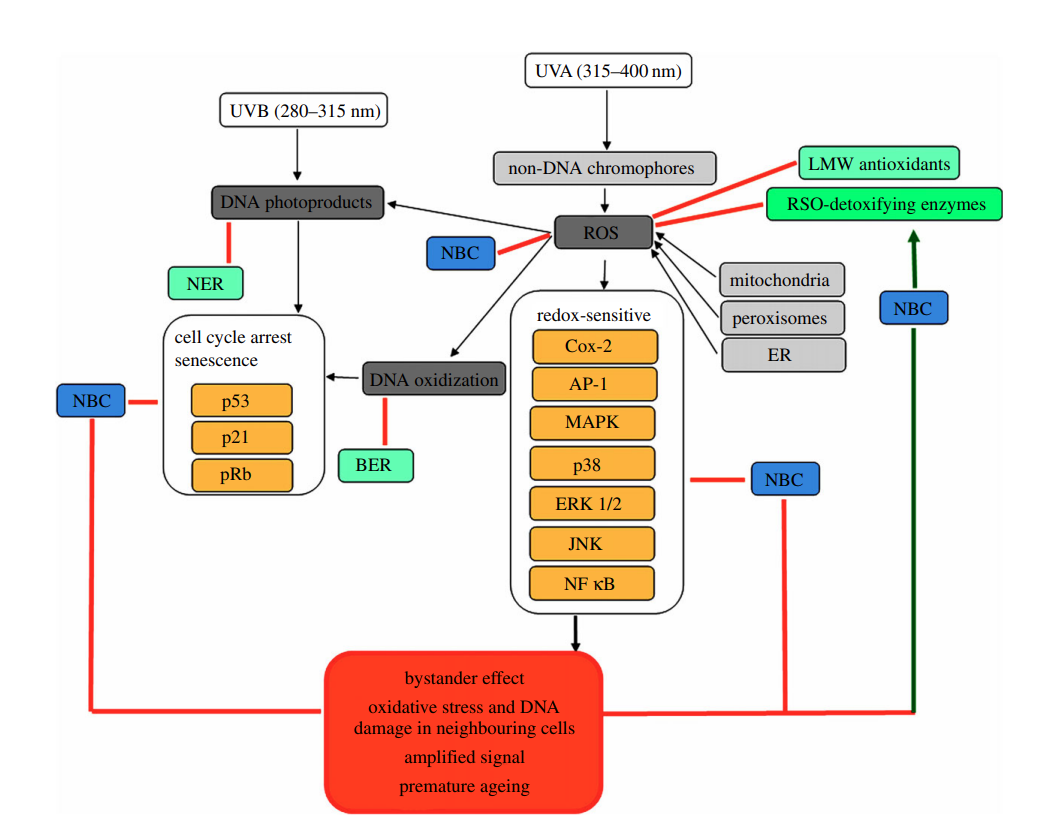
Figure 1 illustrates the proposed capacities of natural bioactive compounds in protecting against UVR-induced DNA damage in the skin.
Antioxidant Defenses of the Skin
The skin is a stratified organ with an endocrine function, providing protection against environmental damage. It has two main layers: the epidermis and dermis. The epidermis contains keratinocytes organized into layers. The stratum corneum, the outermost layer, contains high concentrations of low molecular weight (LMW) antioxidants like vitamin E, vitamin C, ubiquinol, uric acid, and glutathione. Glutathione (GSH) is a key antioxidant that reacts with ROS.
LMW antioxidants and cysteine-rich small proline-rich proteins (SPRRs) counteract increased ROS levels and DNA damage in the stratum corneum. The epidermis also contains ROS-detoxifying enzymes, highly concentrated in the stratum granulosum, which lower ROS levels. The dermis has lower concentrations of ROS and antioxidants.
Communication between the dermis and epidermis is vital for epidermal progenitor cell activity. Changes in the epidermis due to DNA damage and oxidative stress can decrease cellular turnover rate and affect skin barrier function.
The Bystander Effect and Natural Compounds
UVR-induced cellular damage triggers molecular responses in both directly affected and neighboring, non-irradiated cells, through bystander signaling. This effect involves molecular signaling interaction between irradiated and non-irradiated cells. In human skin, the UVR-induced bystander effect is thought to be propagated by inflammatory responses and ROS, causing further oxidative damage and genomic instability in non-exposed cells. This mechanism relies on multiple signaling cascades involving redox and cell cycle signaling molecules.
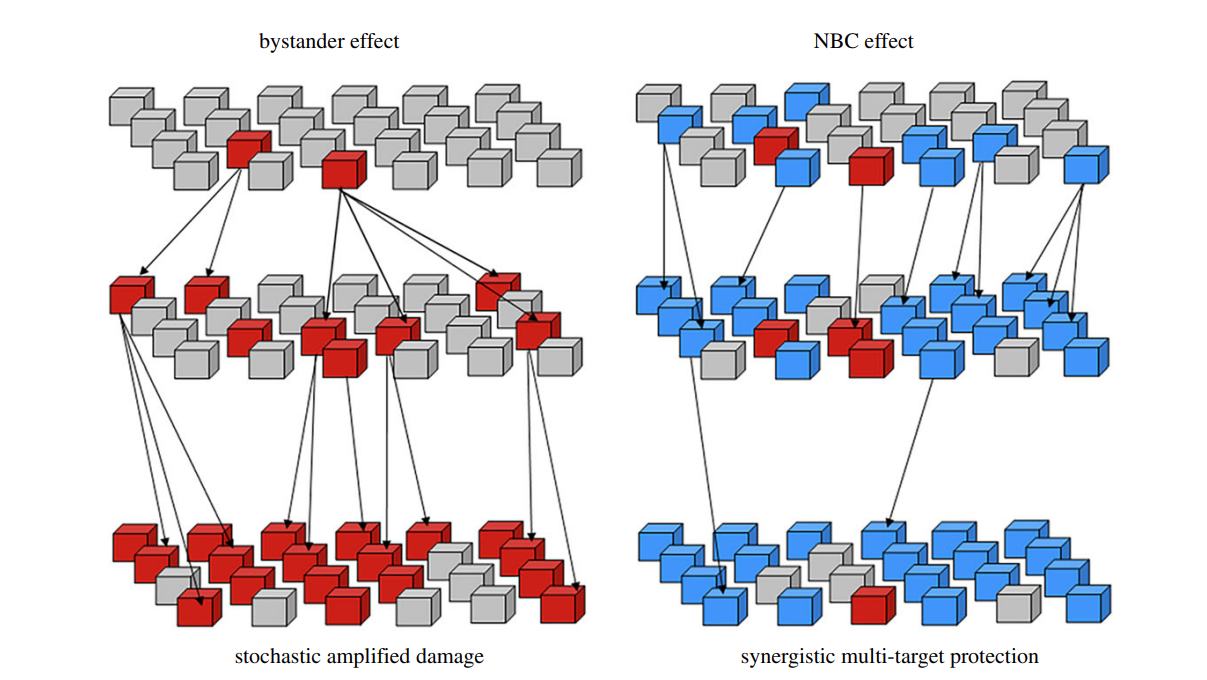
Figure 2 shows interactions between bystander signaling and natural bioactive compounds.
Natural bioactive compounds, secondary metabolites from plant sources, have protective capacities to counteract oxidative damage. They have ROS-scavenging activity and complex interactions with DNA damage and oxidative stress biomarkers. These compounds can act synergistically on multiple molecular targets, affecting several biological pathways simultaneously. Appropriate selection and validation of various natural bioactive compounds may enhance the balance within tissue, including DNA damage levels and bystander signaling.
Natural bioactive compounds like flavonoids, monoterpenes, and polyphenols can inhibit redox-sensitive signaling. Phenolic diterpenes, catechins, and phenolic acids can also increase the activity of ROS-responsive transcription factors and ROS-detoxifying enzymes. Isoflavones and phenolic acids can inhibit various factors while activating others. Diterpenes, flavonoids, and polyphenols can downregulate oxidative stress markers along with cell cycle/DNA damage proteins.
Plant tissue contains secondary metabolites that play a role in defense and tolerance to environmental stresses that can lead to excessive ROS production. The antioxidant properties of these compounds are determined by their chemical structure.
These compounds may regulate activities and repair mechanisms triggered by UVR-induced direct DNA damage and secondary redox-related cellular responses. Natural bioactive compounds could inhibit proteins involved in cell cycle arrest and senescence, enhancing DNA repair, activate ROS-detoxifying enzymes, enhance antioxidant defenses, and inhibit redox-sensitive signaling molecules.
Polypharmacology and Bystander Signaling
In addition to DNA damage upon direct cellular exposure, radiation’s biological effects include non-DNA-targeted effects like the bystander effect. The bystander effect involves damage in unirradiated cells due to interactions with irradiated cells. It amplifies the original damage.
The polypharmacology nature of natural bioactive compounds, their ability to affect multiple protein targets simultaneously, could modulate bystander signaling. These compounds could work in an integrated system to enhance damage recognition and repair.
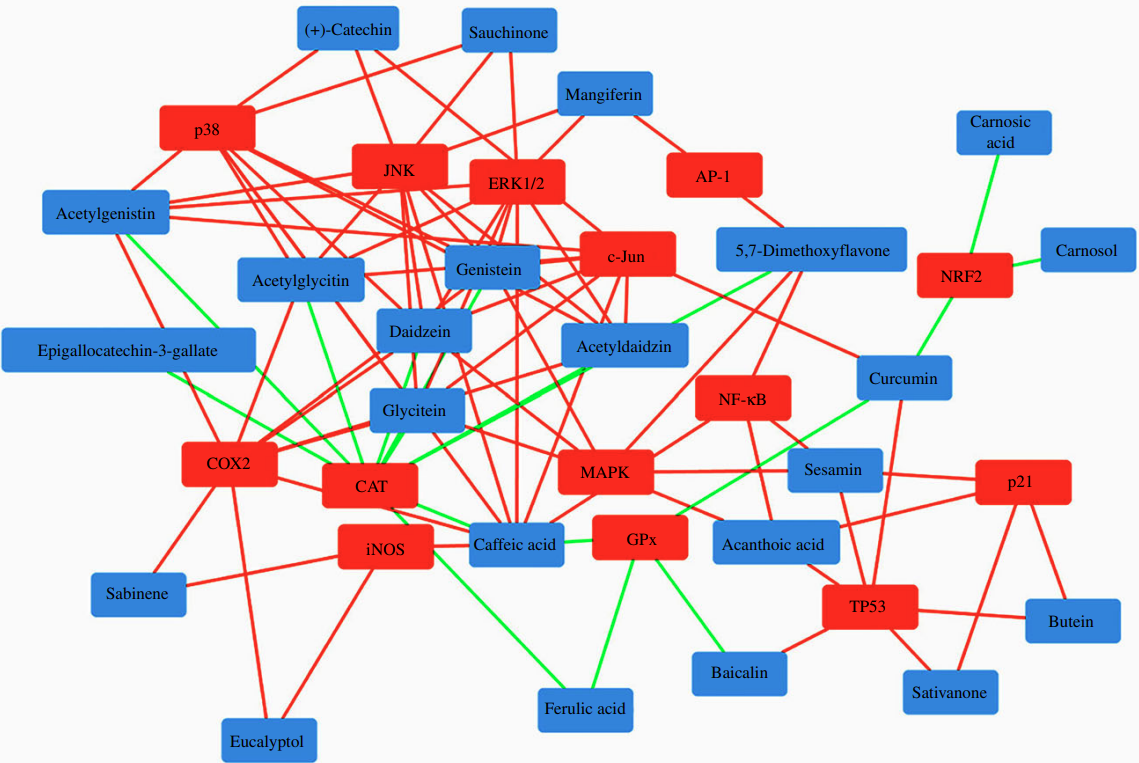
Figure 3 demonstrates interactive networks between natural bioactive compounds and skin cellular targets involved in DNA damage response and redox homeostasis.
Conclusion
UVR is a major factor linked to DNA damage in skin cells. Natural compounds can inhibit the expression or activity of factors involved in the bystander effect, while improving antioxidant and DNA repair capacities.
Sources and related content
[1] – Markiewicz E, Idowu OC.2019 DNA damage in human skin and the capacities of natural compounds to modulate the bystander signalling. Open Biol. 9: 190208. Publications