Cell senescence is an irreversible state of growth arrest, at which a cell permanently exits the cell cycle. This prevents cell division but does not cause apoptosis (Regulski, 2017). This process is influenced by various exogenous or endogenous stressors, including reactive oxygen species (ROS), physical or chemical DNA damaging agents, and some oncoproteins (Abbadie et al., 2017). Because senescence plays a central role in cell senescence and skin ageing, understanding its triggers is essential for developing cellular senescence in skincare approaches. Despite these cells having potential roles in tumour suppression and tissue fibrosis protection, senescent cell accumulation with age can aid in promoting age-related disease.
There are upwards of 5 major markers of cell senescence (Abbadie et al., 2017). Cell enlargement is associated with senescence since these cells continue to accumulate biomass despite not dividing. Cell cycle arrest occurs, typically in G1 phase. This is prolonged and irreversible. Senescent cells are seen to have increased lysosome activity due to increased lysosomal mass. Epigenetic changes to these cells, such as senescence-associated heterochromatic foci (SAHF), aim to stabilise the cells by preventing gene transcription. Secretosome composition changes are also observed, due to the increase of proinflammatory cytokines and extracellular matrix remodellers. This is known as the senescence-associated secretory phenotype (SASP), a major contributor to inflammation-driven ageing and a key concept in anti-ageing molecular skincare.
Accumulation of DNA damage drives skin cell senescence, and senescent cells produce inflammatory cytokines which disrupt tissue structure and alter tissue homeostasis (Ho and Dreesen, 2021). SASPs secreted by these cells can induce senescence in neighbouring cells, resulting in accumulation of dysfunctional cells that can no longer play a role in tissue regeneration and repair (Ho and Dreesen, 2021). Prevention of skin DNA damage, especially UV-induced DNA damage skin, will most likely prevent cells prematurely acquiring a senescent state. Senescent-inducing skin damage is typically caused by photodamage from UV light, causing reactive oxygen species (ROS) formation which damages telomeric and non-telomeric regions of DNA (Ho and Dreesen, 2021). To prevent premature cell ageing, molecular pathways that control cell proliferation must be studied, as deepening an understanding of this will enable cosmetic products to be produced that aid to prevent cell senescence through targeted anti-senescence skincare ingredients.
Cellular proliferation is prevented in senescent cells due to cell cycle arrest typically in the G1 phase. Therefore, these pathways can be targeted in cosmetic skincare, especially within cell proliferation pathways cosmetics research. The basic helix-loop-helix (bHLH) proteins are a family of transcriptional regulators that drive cell types towards differentiation and away from proliferation (Zebedee and Hara, 2001). The retinoblastoma protein (pRB) was the first tumour suppressor to be discovered and plays a role in regulating the cell cycle via downregulating cellular transition from the G1 phase to the S phase (Talluri and Dick, 2012). Because of its regulatory role, the retinoblastoma protein (pRB) in skincare has become an emerging area in molecular anti-ageing strategies. Due to both of these vital proteins negatively regulating proliferation, these can be targeted with specific active ingredients to downregulate their action. This downregulation of bHLH and pRB naturally occurs through the action of cyclin-dependent kinases (CDKs), and inhibitor of differentiation (Id) proteins (Zebedee and Hara, 2001). Upregulating these proteins is key to promoting cell proliferation, and Id proteins and skin regeneration have gained increasing attention for this reason.
Figure 1:
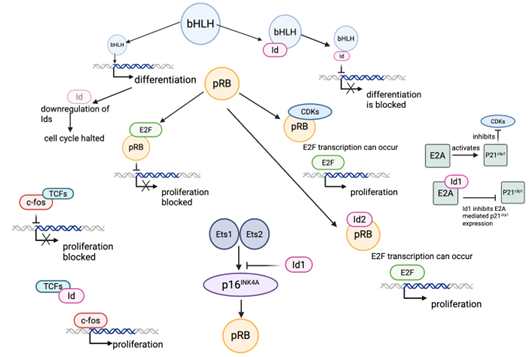
Figure 1: The Retinoblastoma (pRB) tumour suppressor pathway and inhibitors of this, specifically the role of inhibitor differentiation (Id) proteins (Zebedee and Hara, 2001). Id proteins are vital proteins that induce cellular proliferation. Id proteins can inhibit bHLH, blocking its action and driving processes away from cellular differentiation and towards cellular proliferation. pRB is a tumour suppressor which negatively regulates proliferation by binding to E2F transcription factors to prevent their action. pRB can be regulated by CDKs, which competitively inhibit pRB by blocking their E2F binding sites. CDKs are regulated by cyclins and E2A. Activated E2A stimulates P21clip1 to inhibit CDK activity. Id2 binds to pRB to prevent inhibition of proliferative gene transcription. Id1 binds to E2A, preventing activation of P21clip1. Id1 also inhibits the action of Est1 and Est2 in activating p16INK4A. This prevents activation of pRB. Id proteins also possess the ability to competitively bind to TCFs, enabling c-fos transcription factors to transcribe genes promoting proliferation (Zebedee and Hara, 2001).
It is vital to understand natural pathways which prevent cell senescence in order to investigate cell senescence prevention, as active ingredients that affect these pathways are key to creating products with natural ingredients that can actively promote cell proliferation and decrease likelihood of premature cell senescence. This provides the foundation for modern anti-senescence skincare ingredients and the development of advanced anti-ageing molecular skincare.
Sources and related content
[1] – Abbadie, C.; Pluquet, O.; Pourtier, A. Epithelial cell senescence: an adaptive response to pre-carcinogenic stresses? Cellular and Molecular Life Sciences 2017, 74, 4471–4509.
[2] – Ho, C.Y.; Dreesen, O. Faces of cellular senescence in skin aging. Mechanisms of Ageing and Development 2021, 198, 111525.
[3] – Regulski, M.J. Cellular senescence: what, why, and how. Wounds: A Compendium of Clinical Research and Practice 2017, 29, 168–174.
[4] – Talluri, S.; Dick, F.A. Regulation of transcription and chromatin structure by pRB: here, there and everywhere. Cell Cycle 2012, 11, 3189–3198.
[5] – Zebedee, Z.; Hara, E. Id proteins in cell cycle control and cellular senescence. Oncogene 2001, 20, 8317–8325.
