What is Melanin?
Melanin is a class of pigments produced by melanocytes which are cells predominantly found in the basal layer of the epidermis. In humans, we can see the effect of melanin clearly as greater concentrations of melanin is a determinant for darker skin. Melanin is produced by the process of melanogenesis which is controlled by different molecules which interact with each other via pathways, which can be receptor dependent or independent, to regulate melanin production. Melanin is conserved in many species but its main function in humans is in protection against ionising ultraviolet light radiation (UVR) [1]. This is due to its ability to absorb light and dissipate it in the form of heat and their ability to neutralise ionised molecules. The type and amount of melanin that we produce is genetically predetermined but can be influenced by factors such as aging and UV exposure by affecting the signalling pathways responsible for melanogenesis. As skin ages, the epidermis thins, with the number of melanocytes decreasing and remaining melanocytes increasing in size. The study of melanogenesis allows the identification of key components of the pathway and compounds that can stimulate melanogenesis in aging skin.
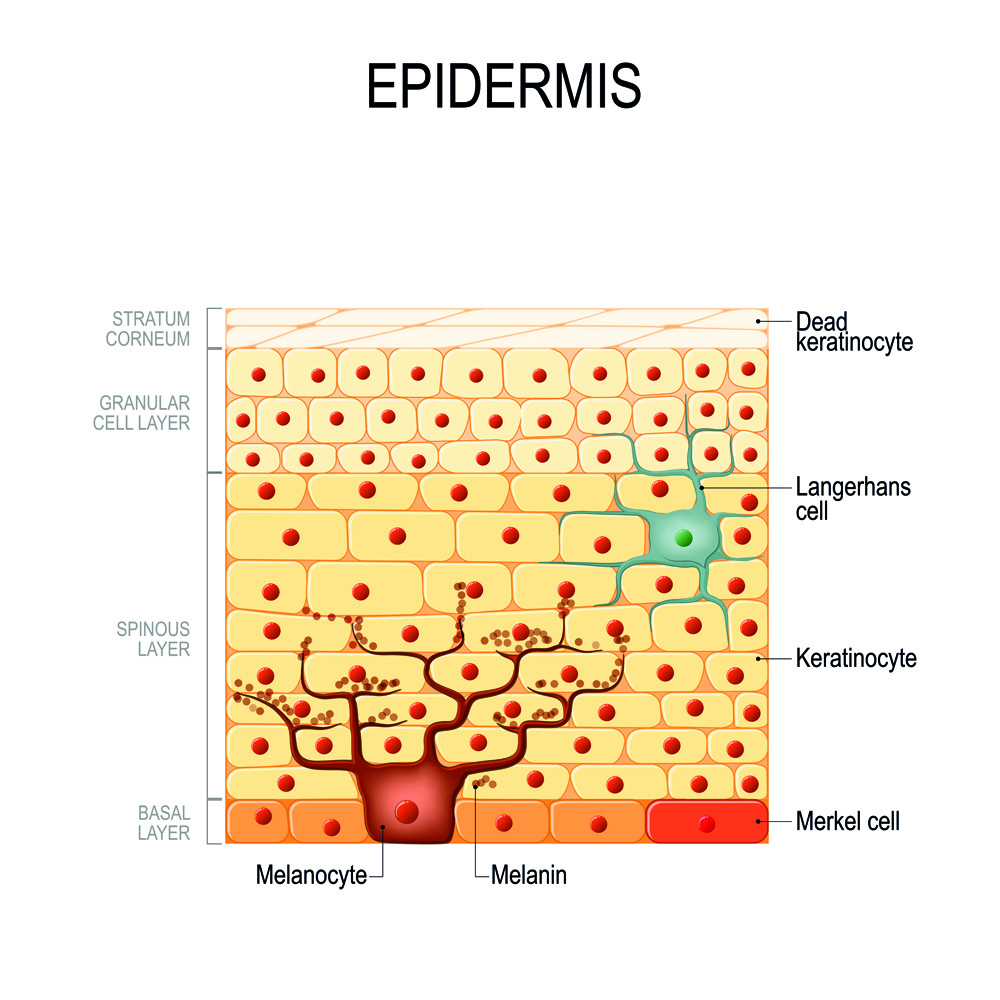
Figure 1. Epidermal layers of the skin in humans showing the position and structures of the melanocytes in the basal layer.
Eumelanin and Pheomelanin
The two main types of melanin pigment required for skin pigmentation are eumelanin and pheomelanin. Melanocytes produce melanosomes (intracellular organelles) which are the site of pigment synthesis. The melanosomes are then transferred to adjacent keratinocytes in the epidermis where the melanin granules accumulate, causing visible skin pigmentation. They protect the cells of the epidermis from UV light (UVA, UVB, and UVC). UVR primarily alters nucleotide structure of cellular DNA through the generation of reactive oxygen species (ROS) [2]. In particular, eumelanin is a potent absorber of UV radiation, so blocks UV from penetrating deeper skin layers, protecting proliferating cells which can have their DNA affected by UVR. However, pheomelanin has be seen to promote carcinogenesis, leading to skin cancer in individuals with a higher percentage of pheomelanin. It has been suggested that this is due to the production of ROS when pheomelanin is irradiated with UVA [3]. Greater density of eumelanin will lead to darker skin, whereas higher concentrations of pheomelanin result in lighter skin (eumelanin to pheomelanin ratios contribute significantly to skin colour). Therefore, it’s been shown that individuals with darker skin are less likely to develop skin cancer than lighter skinned individuals do their increased ability to scatter UV radiation.

Figure 2. Image of the different types of skin tones seen in the population. Darker skin is generated due to a higher concentration of eumelanin, whereas lighter skin is produced through a greater density of pheomelanin.
Melanogenesis Pathways
Melanogenesis is a regulated process, controlled through various agents and factors. The earliest rate-limiting step in this process is the hydroxylation of L-tyrosine to L-DOPA which is catalysed by tyrosine hydroxylase activity of tyrosinase [4]. Thus, the presence of L-tyrosine is essential to produce melanin and is a key target in the control of melanogenesis. Once L-DOPA has been synthesised, subsequent steps in the process can occur spontaneously, reliant on hydrogen ion, oxygen, and metal cation concentration. Melanogenesis-related enzymes (MREs) are essential in regulating velocity and specificity of the pathway. Tyrosinase is an MRE that’s fundamental to this process. It’s encoded by the TYR locus; mutations in this gene result in a form of albinism, demonstrating its importance in melanogenesis [5].
Melanocortin 1 receptor (MC1R) is a G-protein coupled receptor found on the membranes of melanocytes, whose signalling is essential in initiating and regulating melanogenesis as well as in adaptations of the skin to UV exposure. It is activated by its agonists α-melanocyte-stimulating hormone (α-MSH) and adrenocorticotropic hormone (ACTH) which are responsible for eumelanin synthesis, or by agouti signalling protein (ASP) which is responsible for pheomelanin synthesis. Activation of MC1R by α-MSH/ACTH results in increased cAMP production which results in eumelanin production, rather than pheomelanin [6]. Eumelanin is associated with UV radiation protection, whereas pheomelanin has been associated with the development of skin cancer. Mutations in MC1R can be associated with lighter skin, increased risk of melanomas and nonmelanoma skin cancer [7].
TYRP1 and TYRP2 (tyrosinase-related proteins) are found in melanosomes and function to catalyse reactions favouring eumelanin production. TYRP1 is suggested to be most important in eumelanogenesis due to its lack of expression in cells conducting pheomelanogenesis [8]. It has also been shown that TYRP1 stabilises tyrosinase activity, so is essential for melanogenesis. α-MSH upregulates TYRP2 activity and expression whereas it is downregulated by ASP, suggesting that TYRP2 is a positive regulator of eumelanogenesis. This is supported by experiments using mouse models which displayed genetic defects at the TYRP2 locus, resulting in a decreased ratio of eumelanin: pheomelanin [6].
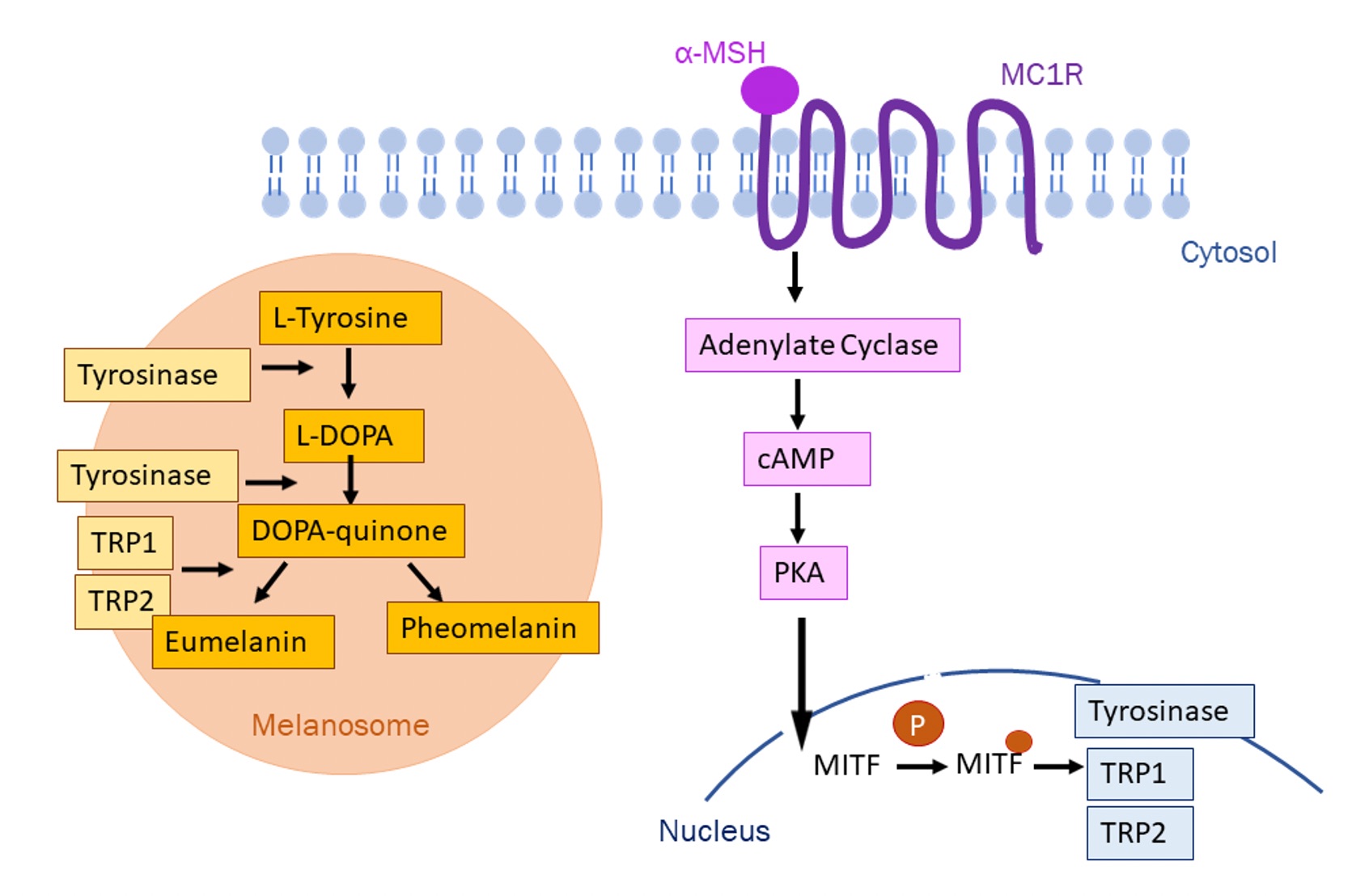
Figure 3. Melanogenesis pathway showing the signalling pathway when melanocortin 1 receptor is activated by α-melanocyte-stimulating hormone and the corresponding effects in the melanosome.
Skin Cancer
Skin cancer is the most prevalent cancer in humans, and it’s reported to be on the rise. It can be divided into two categories: non-melanoma and melanoma skin cancer. Melanomas are defined as an uncontrollable growth of melanocytes. The uncontrolled growth results in overproduction of melanin, due to upregulated melanogenesis, leading to the dark, asymmetrical appearance seen in melanomas. Of all skin cancers, only 4% are diagnosed to be melanoma yet they’ve been shown to be the deadliest form as they account for under half of all mortalities from skin cancer [9]. It was estimated that 60-70% of melanomas are caused by UVR (the most common environmental carcinogen), primarily by UVA and UVB radiation [10]. UVA has a longer wavelength and penetrates deeper layers of the skin, but UVB has been shown to be more genotoxic, which is due to its shorter wavelength. The shorter the wavelength of UVR, the greater potential to cause damage [11]. UVB damages DNA directly via photoproducts such as cyclobutene pyrimidine dimers which can block DNA replication and transcription. These dimers are normally removed in healthy individuals via a nucleotide excision repair (NER) pathway which recruits repair machinery to the site of DNA damage. If this pathway becomes dysregulated or dysfunctional, DNA damage caused by UVB will not be repaired, which may ultimately lead to the production of cancerous skin cells. NER dysfunctionality can also contribute to development of Xeroderma pigmentosum, which is a disease characterised by extreme sun sensitivity. Sufferers of the disease are prone to severe sunburn and blistering from small amounts of UVR. As a result, their risk of skin cancer is massively increased [12]. UVA on the other hand leads to production of ROS when pheomelanin is irradiated. ROS cause DNA damage through oxidising nucleotide bases and these mutations have the potential to be carcinogenic [13].
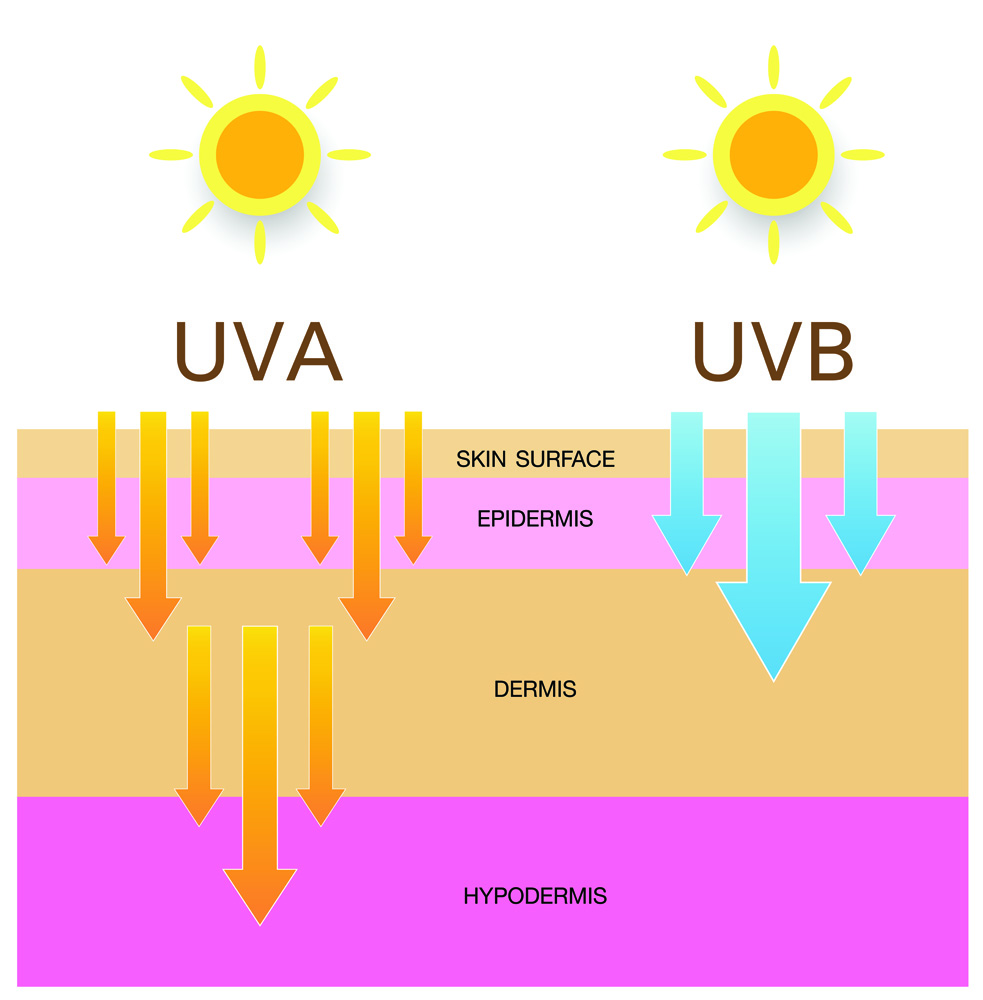
Figure 4. The difference between penetration ability into deeper skin layers of UVA and UVB. As UVA penetrates deeper, it has more potential for SNA damage, so can irradiate pheomeloanin, causing the production of reactive oxygen species. This DNS damage can potentially lead to skin cancer.
Benefits of Sunscreen
The increase in incidence of skin cancer has demonstrated the importance of applying sunscreen. Sunscreens contain compounds that block UVR, with the focus being to block UVA and UVB epidermal penetration. Common active chemical compounds include oxybenzone and avobenzone. These compounds absorb high-intensity UVR, so become excited by the energy before ultimately converting the UVR to lower energy wavelengths such as heat. Physical compounds that can be included in sunscreen are titanium dioxide and zinc oxide. These act to reflect or refract UVR but have been shown to be able to act as semiconductor metals by absorbing UVR. Ultimately, ingredients in sunscreen act to prevent UVR absorption by cells, to prevent DNA damage and the development of skin cancer [11].
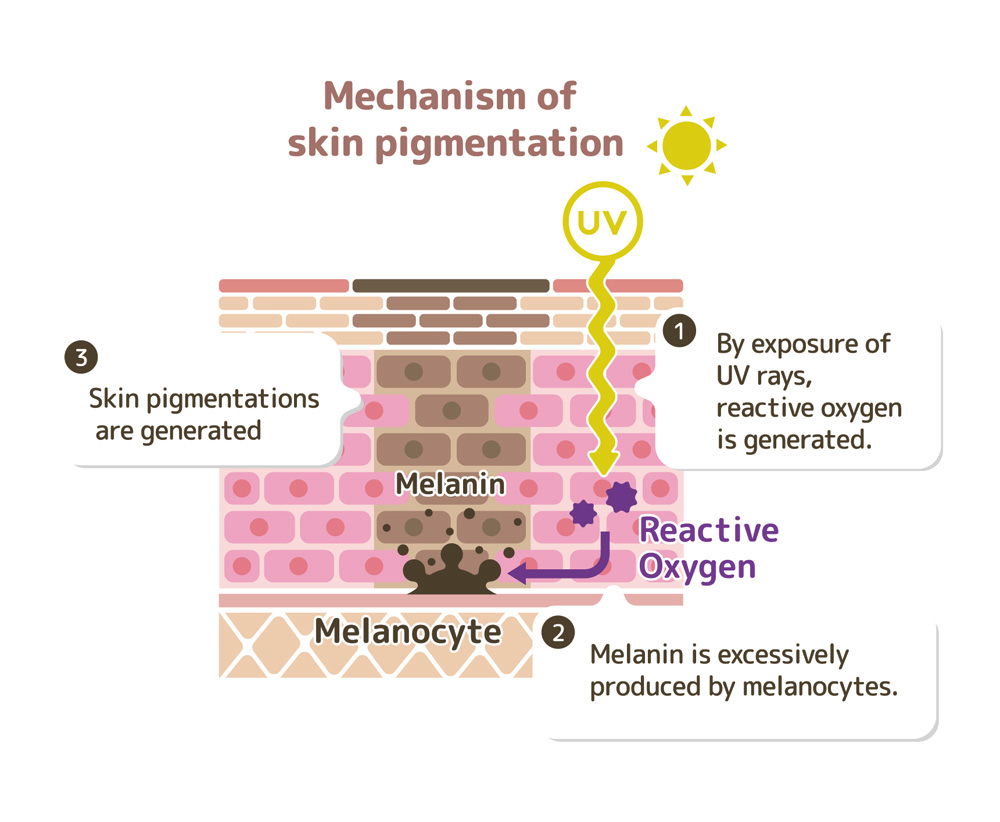
Figure 5. Diagram of how melanin is produced in response to UV radiation to protect the skin from SNA damage from radiation and reactive oxygen species. This is the mechanism of tanning.
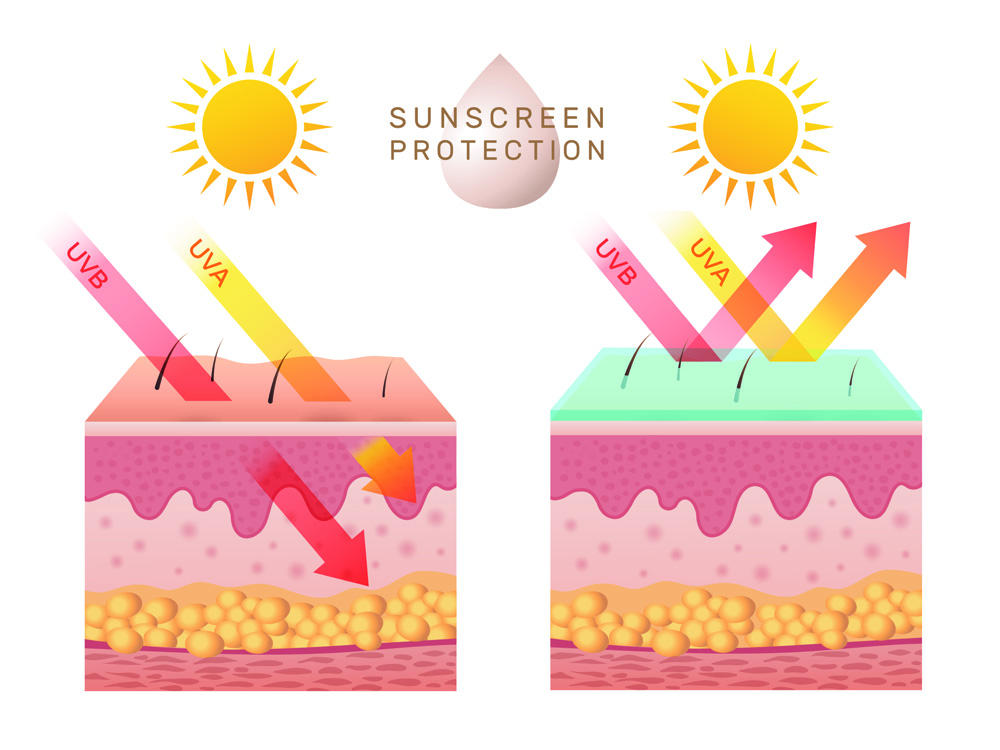
Figure 6. Image showing effect of sunscreen on UV penetration ability into epidermal layers of skin. Sunscreen is essential in preventing penetration, and thus protecting the skin from UV damage.
Adverse Effects of Sunscreen
Despite sunscreen being extremely important in protecting against UVR and thus preventing DNA damage, it has been shown that some of the active ingredients in sunscreens are harmful in themselves. Avobenzone is widely used in sunscreens but has been deemed unstable in physical sunscreens. When exposed to chlorine, avobenzone can react with the disinfectants, leading to the production of toxic by-products. The full extent of the toxicity of these products is unknown, but compounds such as chlorinated phenols and acetophenones which are produced are known to be toxic [14]. This poses a major problem that requires more investigation to determine how safe our current sunscreens are. Thus, there is a demand for more natural ingredients which can offer UVR protection as well as be stable in physical sunscreens to protect us from any harmful by-products.
Skin Lightening
Sunscreen acts to block UVR, to prevent upregulation of melanogenesis in melanoma. However, skin lightening products are available that function through inhibiting melanogenesis. They are available commercially and are typically used cosmetically to alter the appearance of blemishes and darker patches of skin. They can also be used clinically in the treatment of skin disorders such as melasma, which is a condition characterised by dark patches of skin, typically on the face. Melasma is a common condition, more prevalent in darker skinned individuals, and can be caused by UV exposure, stress, and oestrogen and progesterone sensitivity which puts those on birth control or pregnant women at a higher risk of developing melasmas. The two main skin lightening techniques used are topical skin creams and laser treatment. Topical skin creams can range in their potency and use hydroquinone and corticosteroids as their active ingredient. Hydroquinone inhibits melanogenesis via the inhibition of tyrosinase, which thus inhibits the hydroxylation of L-tyrosine to form L-DOPA [15] whereas corticosteroids function through non-selective suppression of melanogenesis [16]. Laser therapy is a more robust approach to skin lightening and becomes an option when patients are resistant to topical creams. Laser or light therapies remove the outer layer of dead skin and can damage melanocytes, depending on their wavelength, thus reducing the production of melanin, leading to overall skin lightening. Cosmetically, these procedures can benefit the mental health and physical appearance of a patient, but the inhibition of melanogenesis comes at a cost. With the main function of melanogenesis being to produce pigments protecting against UVR, downregulating melanogenesis will cause users to be more susceptible to UVR damage and thus at a higher risk of developing skin cancer. It is therefore important for patients who undergo these therapies to use sunscreen as frequently as possible as a preventative measure for the development of melanoma and other skin cancers.
Albinism and Vitiligo
An untreatable condition of the skin related to melanogenesis is albinism which can present in different forms depending on the genes involved. Oculocutaneous albinism (OCA) is a group of 4 autosomal recessive disorder leading to a complete lack or reduction in melanogenesis; this phenotypically presents as hypopigmentation of the skin (as well as the hair and eyes). Patients presenting with albinism have a normal profile of melanocytes, but melanin pigments are absent. For example, mutations in the TYR gene (produces tyrosinase) leads to OCA1 (form of albinism). The mutated tyrosinase cannot catalyse (or catalytic activity is reduced) the hydroxylation of L-tyrosine to L-DOPA meaning that melanin pigments cannot be produced [17]. Unlike in melasmas, the mutation is systemic which leads to the pale, white appearance of sufferers. The lack of melanin means that those with OCA are more susceptible to DNA damage, caused by UVR. As there is no cure for albinism due to its genetic nature, treatments focus on protecting the skin from UV damage with sunscreen, as well as monitoring the skin for any sign of skin cancer [18]. Otherwise, sufferers can lead a normal life.
Vitiligo is another skin disorder, similar to albinism in the sense that there’s depigmentation of the skin, but it doesn’t present as severely, with only patches of the skin being affected. It can be divided into two subcategories: segmented and non-segmented vitiligo. Non-segmented vitiligo is more common and is characterised clinically by multiple affected areas of depigmentation across the body, whereas segmented vitiligo involves one area of the skin. Vitiligo a disorder is a result of the selective loss of melanocytes, leading to pigment dilution in affected areas. There have been multiple causes identified for the destruction of melanocytes including genetic, autoimmune responses, and oxidative stress [19]. It has been suggested that multiple mechanisms work together to ultimately result in vitiligo. Due to the melanocyte destruction, melanogenesis is downregulated, leading to a weaker concentration of melanin pigments in the skin. As with albinism, the patches of affected skin are susceptible to UV radiation and thus DNA damage. Vitiligo is incurable but has a broader range of treatment options than with albinism. If diagnosed early, topical corticosteroids can be prescribed. Despite being used as an active ingredient in skin lightening agents, corticosteroids also have potent anti-inflammatory and immunosuppressive properties, so are used to slow the mechanism of melanocyte destruction, and thus vitiligo development [20]. However, the importance of sunscreen to prevent DNA damage by UVR is an essential treatment.
Effects of Aging
As the skin ages, the number of melanocytes in the epidermis decreases, leading to an uneven distribution, affecting how melanocytes interact with keratinocytes to produce skin pigmentation. Due to the loss of melanocytes, when stimulated by UVR, remaining melanocytes will continue to produce pigment, but due to their isolation, the pigment will accumulate over time and present as a spot of hyperpigmentation. These are referred to as age spots and are light brown/black pigmented lesions. Age spots are associated with skin that has been chronically exposed to UVR, but their exact mechanism of production still isn’t well known [21]. Skin lightening agents can be used to try and reduce the appearance of age spots, typically in older women.
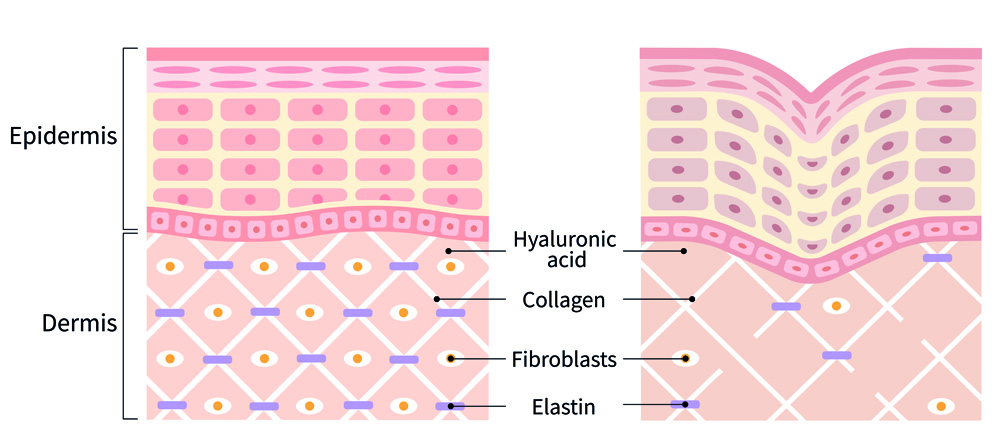
Figure 8. Difference between young skin (left) and older skin (right). Image shows the reduced content of the dermis, so the skin has less support, meaning it’s less firm, and more prone to wrinkles.
Conclusions and Recommendations
Melanin is essential in the protection of our skin from harmful UVR and thus in the protection of our DNA from UVR-induced DNA damage. Without melanin, it’s shown that we’re at higher risks of developing skin cancers including melanomas which are aggressive and life-threatening. Active ingredients such as oxybenzone and avobenzone are also useful in protecting us from DNA damage from UVR but have also been shown to be toxic (mainly avobenzone). The foundational basis of sunscreens thus needs to be rewritten, to create a formulation effective at UV protection, without the added toxicity. Melanin’s function in protecting us from UVR is essential, but it can be necessary to downregulate melanogenesis in skin cosmetics where hyperpigmentation of melanin causes dark patches of skin. The downregulation by ingredients such as hydroquinone and corticosteroids can correct the appearance of darker spots, but also result in decreased protection from UVR. Thus, sunscreen has been shown to be essential to protect the skin, especially in those who may have their protection somewhat compromised.
References
[1] – Ahene AB, Saxena S, Nacht S. Photoprotection of solubilized and microdispersed melanin particles. Investigative Dermatology. 1994 Feb 1 (Vol. 102, No. 2, pp. 268-268). [2] – Maresca V, Flori E, Briganti S, Camera E, Cario-André M, Taïeb A, Picardo M. UVA-induced modification of catalase charge properties in the epidermis is correlated with the skin phototype. J Invest Dermatol. 2006 Jan;126(1):182-90. doi: 10.1038/sj.jid.5700021. [3] – Morgan AM, Lo J, Fisher DE. How does pheomelanin synthesis contribute to melanomagenesis?: Two distinct mechanisms could explain the carcinogenicity of pheomelanin synthesis. Bioessays. 2013 Aug;35(8):672-6. doi: 10.1002/bies.201300020. [4] – Hearing VJ, Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991 Nov;5(14):2902-9. [5] – Oetting WS, King RA. Molecular basis of albinism: mutations and polymorphisms of pigmentation genes associated with albinism. Hum Mutat. 1999;13(2):99-115. doi: 10.1002/(SICI)1098-1004(1999)13:2<99::AID-HUMU2>3.0.CO;2-C. [6] – Borovanský J., Wiley I. Melanins and Melanosomes Biosynthesis, Biogenesis, Physiological, and Pathological Functions. John Wiley & Sons. 2011 Aug 15. [7] – Valverde P, Healy E, Sikkink S, Haldane F, Thody AJ, Carothers A, Jackson IJ, Rees JL. The Asp84Glu variant of the melanocortin 1 receptor (MC1R) is associated with melanoma. Hum Mol Genet. 1996 Oct;5(10):1663-6. doi: 10.1093/hmg/5.10.1663. [8] – del Marmol V, Ito S, Jackson I, Vachtenheim J, Berr P, Ghanem G, Morandini R, Wakamatsu K, Huez G. TRP-1 expression correlates with eumelanogenesis in human pigment cells in culture. FEBS Lett. 1993 Aug 2;327(3):307-10. doi: 10.1016/0014-5793(93)81010-w. [9] – Guy GP Jr, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015 Feb;48(2):183-187. doi: 10.1016/j.amepre.2014.08.036. [10] – Koh HK, Geller AC, Miller DR, Grossbart TA, Lew RA. Prevention and early detection strategies for melanoma and skin cancer. Current status. Arch Dermatol. 1996 Apr;132(4):436-43. [11] – Sander M, Sander M, Burbidge T, Beecker J. The efficacy and safety of sunscreen use for the prevention of skin cancer. CMAJ. 2020 Dec 14;192(50):E1802-E1808. doi: 10.1503/cmaj.201085. [12] – Bradford PT, Goldstein AM, Tamura D, Khan SG, Ueda T, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet. 2011 Mar;48(3):168-76. doi: 10.1136/jmg.2010.083022. [13] – Hemnani T, Parihar MS. Reactive oxygen species and oxidative DNA damage. Indian J Physiol Pharmacol. 1998 Oct;42(4):440-52. [14] – Lebedev AT, Bavcon Kralj M, Polyakova OV, Detenchuk EA, Pokryshkin SA, Trebše P. Identification of avobenzone by-products formed by various disinfectants in different types of swimming pool waters. Environ Int. 2020 Apr;137:105495. doi: 10.1016/j.envint.2020.105495. [15] – Sofen B, Prado G, Emer J. Melasma and Post Inflammatory Hyperpigmentation: Management Update and Expert Opinion. Skin Therapy Lett. 2016 Jan;21(1):1-7. [16] – Deo KS, Dash KN, Sharma YK, Virmani NC, Oberai C. Kojic Acid vis-a-vis its Combinations with Hydroquinone and Betamethasone Valerate in Melasma: A Randomized, Single Blind, Comparative Study of Efficacy and Safety. Indian J Dermatol. 2013 Jul;58(4):281-5. doi: 10.4103/0019-5154.113940. [17] – Tomita Y, Takeda A, Okinaga S, Tagami H, Shibahara S. Human oculocutaneous albinism caused by single base insertion in the tyrosinase gene. Biochem Biophys Res Commun. 1989 Nov 15;164(3):990-6. doi: 10.1016/0006-291x(89)91767-1. [18] – Grønskov K, Ek J, Brondum-Nielsen K. Oculocutaneous albinism. Orphanet J Rare Dis. 2007 Nov 2;2:43. doi: 10.1186/1750-1172-2-43. [19] – Ezzedine K, Lim HW, Suzuki T et al; Vitiligo Global Issue Consensus Conference Panelists. Revised classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res. 2012 May;25(3):E1-13. doi: 10.1111/j.1755-148X.2012.00997.x. [20] – Rodrigues M, Ezzedine K, Hamzavi I, et al. Vitiligo Working Group. Current and emerging treatments for vitiligo. J Am Acad Dermatol. 2017 Jul;77(1):17-29. [21] – Choi W, Yin L, Smuda C, Batzer J, Hearing VJ, Kolbe L. Molecular and histological characterization of age spots. Exp Dermatol. 2017 Mar;26(3):242-248. doi: 10.1111/exd.13203